My personal sulphuric bugbear is the odour and flavour from light struck hops, more commonly known as skunking. It irritates me because it is the most easily preventable of all the invasive sulphur tints that plague beer - just stick the bloody beer in a brown bottle. The chemistry of skunking can be seen below. I love organic chemistry, but it's not for everyone and I am sorry for inflicting it on those who do not share my interest. The photolytic reaction to the left/down is the cause of my woes, the result of which is isopentyl mercaptan - the classic skunky smell. The top reaction running left to right is some clever brewing scientist's bright idea to prevent the breakdown of the hop alpha acid and remove the production of unpleasant sulphur
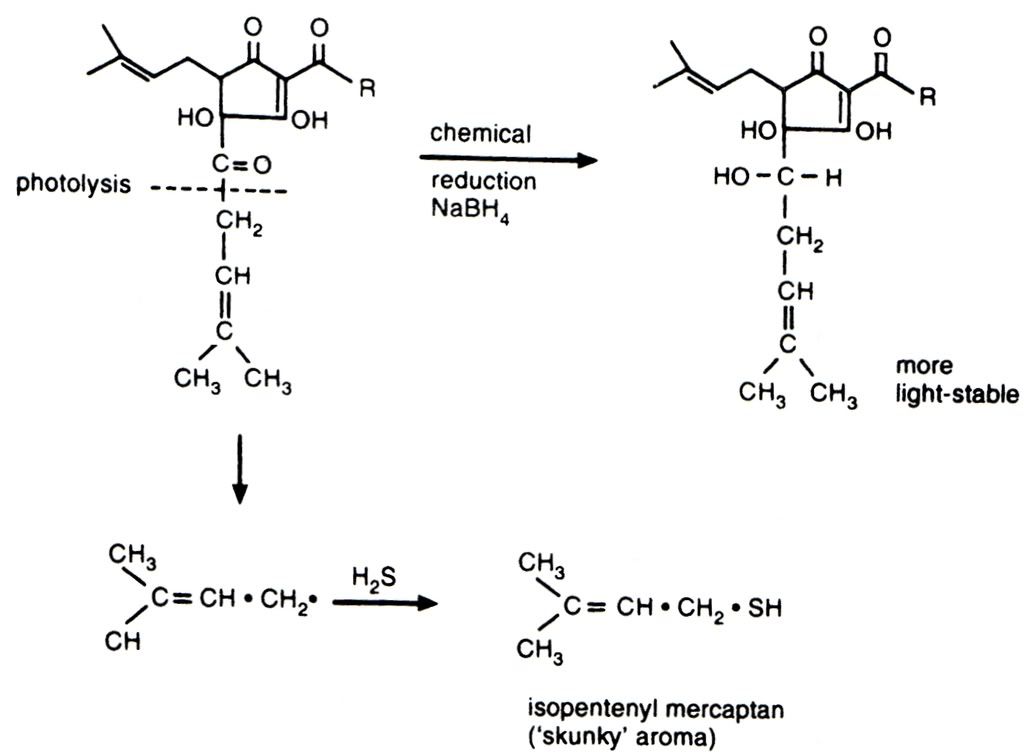 compounds. These products are called reduced alpha acids (reduction is a chemical process involving the donation of electrons to a molecule). These reduced compounds do not suffer from light strike and also have the added bonus of providing more bitterness than an equivalent measure of standard alpha acids. Curiously they also promote far better foam stability, but at the risk of the foam looking rocky and artificial.
compounds. These products are called reduced alpha acids (reduction is a chemical process involving the donation of electrons to a molecule). These reduced compounds do not suffer from light strike and also have the added bonus of providing more bitterness than an equivalent measure of standard alpha acids. Curiously they also promote far better foam stability, but at the risk of the foam looking rocky and artificial.I stopped buying beer in clear bottles quite some time ago because of the unpleasant smell and flavour it suffers from. This is shame because there are quite a number of good beers sold in clear glass bottles. The difference between a beer in its intended state and the sorry condition it reaches us in clear glass has been highlighted to me on a number of occasions. The first was Marston's Old Empire IPA, an unremarkable beer in the bottle that tastes just like every other light struck beer, but a pint of it from cask at th
 e GBBF proved entirely different and not just because of the cask/bottle divide. The most striking difference I have experienced was Bishop's Finger from a bottle that had been kept under wraps away from mischievous photons. It is a true strong malty richly hopped ale in this condition, but tastes like every other ale in a clear bottle when exposed to light. Happily I can say the same for this particular bottle of 1698 because the people at CAMRA must have kept it from the light during processing, and I, hopeful that they had done so, stashed it in the cupboard in the hope that it might be free from light struck off flavours. Thankfully it has not a hint of skunking. Instead it is a wonderful strong ale with full, sticky body and satisfying minerally English hops. I do not know why Shepherd Neame put all their beer in clear bottles. It seems crazy to me, especially with the generous hopping that is typical in most of their ale.
e GBBF proved entirely different and not just because of the cask/bottle divide. The most striking difference I have experienced was Bishop's Finger from a bottle that had been kept under wraps away from mischievous photons. It is a true strong malty richly hopped ale in this condition, but tastes like every other ale in a clear bottle when exposed to light. Happily I can say the same for this particular bottle of 1698 because the people at CAMRA must have kept it from the light during processing, and I, hopeful that they had done so, stashed it in the cupboard in the hope that it might be free from light struck off flavours. Thankfully it has not a hint of skunking. Instead it is a wonderful strong ale with full, sticky body and satisfying minerally English hops. I do not know why Shepherd Neame put all their beer in clear bottles. It seems crazy to me, especially with the generous hopping that is typical in most of their ale.As an aside, in the above photolytic chemical reaction it is the presence of the oxygen atom and double bond that is responsible for light strike. Oxygen is very electronegative which means it really pulls on the electrons in the bond with the lower carbon atom. This weakens the bond and allows the lower fraction of the molecule to be cleaved off by an energetic photon. This same electronegativity is responsible for all life on earth in as much as it is the reason why a small molecule like water is a liquid at ambient temperature. So I'm left with a dilemma; all life on earth or skunk free beer?