Wednesday, September 23, 2009
Hardness and alkalinity Part 1
Hardness: This is essentially the concentration of calcium and magnesium in the water. It is divided into two further sets of terms:
Permanent hardness also called non-carbonate hardness. This cannot be removed from the water by simple means. It is made up from calcium and magnesium compounds such as calcium sulphate, magnesium sulphate, calcium chloride and magnesium chloride.
Temporary hardness also called carbonate hardness or alkalinity. This can be removed through the various treatments that brewers use, and is responsible for the lime-scale on household appliances. It is made up of calcium carbonate and calcium bicarbonate.
The significance of the distinction is seen when we consider what the two types of hardness bring to brewing. Carbonate hardness increases mash pH by neutralising the natural acids contributed by the mash, dragging the mash pH away and above the 5.4 that is optimal for malt amylases to work. Non carbonate hardness lowers mash pH and is beneficial to meeting the optimal mash pH.
The contrasting action of these differing forms of hardness leads us to another important term in brewing water chemistry: residual alkalinity. This is the net effect of the water hardness on the mash, and the extent to which the water will have to be treated to meet requirements. For most Irish water supplies carbonate hardness will out-weigh non carbonate hardness and the water will require a degree of treatment to lower the alkalinity.
Water reports state water hardness in 'equivalents' of calcium carbonate or bicarbonate. This is necessary in order to compare the different types of hardness in the water on a equal footing. When compared in this way it is seen that 3.5 equivalents of permanent calcium hardness or 7.5 equivalents of magnesium hardness is required to offset the pH raising effects of 1 equivalent of carbonate hardness. It is clear from these figures that carbonate hardness is very potent at increasing mash pH. If your water supply is low in carbonate hardness it might be possible to offset the residual alkalinity through the addition of calcium sulphate or calcium chloride, which is likely to be added at any rate in order to increase the calcium concentration to a more suitable level. However, if the carbonate hardness is very high, adding calcium to the water will not be sufficient to overcome the residual alkalinity and the carbonate hardness must be removed.
In my next post I will provide some simple methods for estimating alkalinity, and how to remove it.
Thursday, August 27, 2009
Calcium
- It influences mash pH in a beneficial way, ensuring that the pH is kept low enough for effective mashing. This is achieved through interaction of calcium with carbonates in the water. Carbonates tend to increase wort pH, dragging it away from the optimal pH of around 5.2. Calcium binds to carbonates forming compounds that precipitate out of solution and remove the ability of carbonates to influence mash pH.
- Calcium protects malt amylases against heat inactivation during the mash. Malt amylases steadily lose their ability to convert starch into simpler sugars during the mash because the mash temperature is a compromise between the optimal operating temperature of both alpha and beta amylase. Alpha amylase suffers most during the mash, but a sufficient calcium concentration protects the enzyme from heat inactivation.
- During the boil trub is formed by the precipitation of protein matter through thermal degradation, but calcium plays a significant role in trub formation by neutralising protein molecules through electrostatic interactions. A minimum calcium concentration of 100 mg/l is required for good trub formation.
- Yeast flocculation is aided by calcium through the interaction with proteins on yeast cell walls. Most strains require at least 50 milligrams/litre (mg/l) for good flocculation.
- Beer stone is formed from a build up of oxalate on brewing equipment. Oxalate in packaged beer provided nucleation sites for carbon dioxide that promotes gushing upon opening of the package. Values of 70 - 80 mg/l are sufficient to remove excess oxalate from the brewing process.
When making calcium additions it is important to account for the significant amount of calcium that is retained in the grain during mashing. The calcium that ensures effective mash pH is sacrificed during the acidification process in the form of calcium carbonate, a solid that precipitates and sticks to the grain. This loss can account of or 50 - 60% of the calcium in the mash.
Increasing calcium values to 200 mg/l has shown to increase run off from the mash tun, improve extraction and also increase free amino nitrogen - an essential nutrient for yeast. As the the wort gravity decreases during run off the pH tends to increase, promoting the undesirable extraction of tannins and silica from malt husks. However, it has been noted that increasing calcium in sparge water to 200 mg/l can prevent the wort pH increasing and reduces the extraction undesirable compounds.
The amount of calcium in your brewing water can be measured using test kits that are commonly used for aquarium water analysis. However, it can be assumed that most brewing water does not a have the 100 - 150 mg/l calcium that is desired for brewing. Below are some simple calculations for making calcium additions using minerals commonly used in brewing.
- The water will have a some calcium already present, though it will very likely be sub-optimal. When calculating the amount of calcium to add, the quantity of calcium in the water already must be subtracted. So that's the first step.
- This value is then divided by the percentage of calcium present in the salt you have decided to add. This value differs depending on the salt used and the extent to which water is bonded to it. Calcium sulphate has the chemical formula CaSO4.2H2O. A bit of chemistry tells us that calcium accounts for 23% of this molecule, so the value of calcium in mg/l that we want to add is divided by 0.23.
- This value is then multiplied by the litres of water that you want to treat.
The most common calcium additions in brewing water come from the following salts:
Calcium sulphate: CaSO4.2H2O - 23% of which is calcium
Calcium chloride: CaCl2.
Calcium chloride is very hygroscopic, which means it readily absorbs water from the atmosphere. As a result it is found in a number of forms. This influences the amount of the salt that must be added. If the molecule has two water molecules attached calcium makes up 27% of the molecule, but if has seven water molecules attached calcium makes up only 18% of the molecule. That means our numbers in the calculations in step two are 0.27 and 0.18 respectively.
An example might help clarify this:
Take some tap water with a calcium concentration of 60 mg/l. Let's say we boost the calcium to a more respectable 150mg/l, and we're brewing a stout so we should use calcium chloride to add some body.
There's 60mg/l calcium in the water already, this means we need an additional 90 mg/l to meet our desired value of 150 mg/l. Calcium chloride takes up water readily so we'll assume that your supply of calcium chloride is fully saturated with water thanks to the shoddy way you store it. This means you have:
CaCl2.7H2O - 18% of which is calcium.
You want to add 90mg/l:
You have, say, 30 litres of water to treat, so:
The scenario is the same for the addition of calcium sulphate to water used to brew a crisply hopped pale ale.
We need to add, say, 100 mg/l calcium so,
And we need to treat 40 litres of water:
Saturday, June 27, 2009
Lipids
Lipids take the generic form shown below:
 The simplest form is glycerol and lipids of this structure are called triglycerides. The R group on the left hand molecule represents long chain fatty acids of various lengths. Triglyceride is the storage form of fatty acids and these long chains can be cleaved off the molecule and metabolised when energy is required. The long chains have further variation in the extent to which they are saturated. Saturation refers to the type and number of bonds between the carbon atoms in the chain. Fatty acids are grouped into saturated and polyunsaturated. The classic animal fat that we eat is stearate and looks like this:
The simplest form is glycerol and lipids of this structure are called triglycerides. The R group on the left hand molecule represents long chain fatty acids of various lengths. Triglyceride is the storage form of fatty acids and these long chains can be cleaved off the molecule and metabolised when energy is required. The long chains have further variation in the extent to which they are saturated. Saturation refers to the type and number of bonds between the carbon atoms in the chain. Fatty acids are grouped into saturated and polyunsaturated. The classic animal fat that we eat is stearate and looks like this: It contains 18 carbon atoms with single bonds between each of the atoms and forms a solid at room temperature due to the tendency of the long chains to tangle and clump together. Lard is the classic saturated fatty acid presentation.
It contains 18 carbon atoms with single bonds between each of the atoms and forms a solid at room temperature due to the tendency of the long chains to tangle and clump together. Lard is the classic saturated fatty acid presentation.Unsaturated fatty acids contain double bonds between one or more of the carbons in the chain of the molecule. This prevents the chains from tangling and produces the liquid forms of fat we usually associate with vegetable oils. Linolenic acid is a polyunsaturated fatty acid (PUFA) with 3 double bonds that kink the molecule and prevent packing together of the chains:

Lipids are pesky things in the brewing process. Malt contains about 3.5% lipid material but less than 5% of this material usually makes its way into wort. Mash filters, used by the big time brewers are the worst offenders for this. During the mash filter process the mash is squeezed by an air compressed membrane, removing every last drop of extract from the process but squeezing out a lot of undesirable elements too. This represents the higher end of lipid extraction, while traditional mash tuns can cut this back to around 0.3%.
The presence of lipids is quite obvious; turbid wort contains greater lipid content. This can be clearly seen during the recirculation of wort in batch sparging at home. In general it is beneficial to reduce lipid content in wort, and brewers have differing requirements for the turbidity of the wort they produce. Yeast benefit from lipids in wort and a more vigorous fermentation is often noted, but this must be balanced against the potential problems later in the production line.
Lipid extraction can be increased through a number of ways:
- The use of over modified malt
- A finer malt grind - particularly hammer milling used in mash filters
- Higher mash and sparge temperatures
- Fast wort run off
- Lack of adjuncts
- Squeezing the mash to recover residual extract
Further to this most obvious and common lipid problem is the more obscure interaction of lipids with other malt constituents. For example, amylose - the form of starch found in malt, complexes with lipids forming molecules that are inaccessible to the malt amylase. The unsuccessful break down of malt starch during mashing causes all sorts of problems with extract recovery and, later on, haze in the finished beer. Alterations to beer flavour also stem from lipid interactions through the formation of complexes with esters during fermentation. Esters provide most of the fruity and aromatic flavours in beer that do no stem from hops. These flavours can be diminished by lipid interaction and alter the flavour of the beer.
Thursday, January 8, 2009
The Boil
 A solid rolling boil is essential in the brewing of good beer. It is energy intensive and potentially dangerous but a brewer skimps on boil time or intensity at his/her own peril. The boil must be vigorous and rapid, generally not longer than an hour. The intensity of the boil can be judged by the amount of water evaporated, with a figure of at least 10% being considered the minimum required to achieve what needs to done during the boil. The chemistry involved in wort boiling is immensely complex but can but can be broken down into a number of relatively straight forward mechanisms that contribute to beer quality.
A solid rolling boil is essential in the brewing of good beer. It is energy intensive and potentially dangerous but a brewer skimps on boil time or intensity at his/her own peril. The boil must be vigorous and rapid, generally not longer than an hour. The intensity of the boil can be judged by the amount of water evaporated, with a figure of at least 10% being considered the minimum required to achieve what needs to done during the boil. The chemistry involved in wort boiling is immensely complex but can but can be broken down into a number of relatively straight forward mechanisms that contribute to beer quality.One of the most important roles of the boil brewers carry out is the production of bittering compounds form the isomerisation of hop alpha acids. Isomerisation is a chemical process which involves molecules being converted from one configuration to another. Alpha acids are dubbed iso-alpha acids once isomerised but they contain the same amount of atoms, merely in a different configuration. The isomerisation reaction is favoured by alkaline conditions with a pH of around 9 being optimal, but these conditions are never met during the boil and this explains the notoriously poor level of hop utilisation during the brewing process which rarely exceeds 40%. Wort becomes steadily more acidic during the boil due to the formation of break material so the extraction of bitte
 ring compounds becomes less efficient as the boil goes on. Along with specific pH conditions, magnesium or another divalent ion and a vigorous boil are required to carry out the isomerisation reaction. The gravity of the wort can further influence the isomerisation reaction with high gravity worts impeding the progress of the isomerisation step. The loss of precious bittering compounds is bad enough, but the brewer can expect to further lose what little bittering has been achieved through adsorption to yeast and filter material and also some will be scrubbed by Co2 production during fermentation.
ring compounds becomes less efficient as the boil goes on. Along with specific pH conditions, magnesium or another divalent ion and a vigorous boil are required to carry out the isomerisation reaction. The gravity of the wort can further influence the isomerisation reaction with high gravity worts impeding the progress of the isomerisation step. The loss of precious bittering compounds is bad enough, but the brewer can expect to further lose what little bittering has been achieved through adsorption to yeast and filter material and also some will be scrubbed by Co2 production during fermentation.


Colloidal stability
We all love bright haze free beer and this is one of the major reasons that wort boiling must be carried out properly. During the boil molecules called polyphenols stemming from malt and hops bind to the protein in the wort and form complexes that precipitate out of solution and comprise the hot break material. These processes can take up to 2 hours, but boils rarely exceed 90 minutes for reasons of economy. If the boil is not of sufficient duration to allow break formation, polyphenols and protein matter will persist into the beer and cause problems with clarity. After boiling, wort can contain up to 8000 mg/L of break material which is generally removed before the wort is fermented, though some brewers think that the break material provides additional nutrition for yeast and leave a degree of it in. Kettle finings such as carageenan moss is used to aid precipitation by binding the break material forming large flocs which fall from solution more efficiently than the break material would alone. It should be noted that specific doses of kettle finings are required to get maximum sedimentation and brewers carry out trials to determine the optimal dosage.
Sterilisation
The crushed malt that brewers mash in with is awash with unwanted and deleterious microorganisms such as bacteria and wild strains of yeast. The boiling of wort provides the very important role of sterilising the wort before fermentation lest the unwanted microbes present in the freshly produced wort wreak havoc with the fermentation process.
Enzyme inactivation
The fresh wort drawn from the mash tun is a cocktail of active enzymes that have been hard at work during the mashing process. This is a cause of concern to the brewer who has no do
 ubt carefully selected the grain bill and carried out the mash at a specific temperature with the intention of attaining desired characteristics mainly pertaining to body and residual extract. If mashing enzymes such as the amylases are permitted to continue what they do best in the wort, the residual sugars which the brewer fought to keep in the wort will be degraded and metabolised during fermentation. Beta galactosidase is an enzyme of concern because it breaks down dextrins and makes them accessible to the remaining amylases. This enzyme has been shown to survive mashing and is exploited by distillers - who do not boil their wort - to ensure maximum fermentables are available during fermentation. Distillery fermentations often drop down as low as .997 thanks to the activity of beta galactosidase, so brewers would be best served to stop this enzyme in its tracks before fermentation starts.
ubt carefully selected the grain bill and carried out the mash at a specific temperature with the intention of attaining desired characteristics mainly pertaining to body and residual extract. If mashing enzymes such as the amylases are permitted to continue what they do best in the wort, the residual sugars which the brewer fought to keep in the wort will be degraded and metabolised during fermentation. Beta galactosidase is an enzyme of concern because it breaks down dextrins and makes them accessible to the remaining amylases. This enzyme has been shown to survive mashing and is exploited by distillers - who do not boil their wort - to ensure maximum fermentables are available during fermentation. Distillery fermentations often drop down as low as .997 thanks to the activity of beta galactosidase, so brewers would be best served to stop this enzyme in its tracks before fermentation starts.Volatile Removal
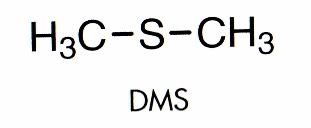
The large plumes of steam that pour from the kettle during the boil carry out the important action of removing with it unwanted volatile compounds that would negatively impact on the flavour of the beer should they be permitted to stay in the wort. These compounds stem from the action of heat on hop constituents and also compounds present in the malt. Unpleasant, harshly bitter hop molecules are driven out in the steam along with dimethyl sulphide (DMS) which is present in lightly kilned malt. DMS is not a major factor during ale brewing because the slightly higher kiln temperature during the production of pale malt drives off most of the DMS. Lager malt has higher levels, but a slight DMS character is considered to be part of the style of European lagers so total elimination during the boil rarely occurs. An important practical consideration with respect to the removal of these compounds is to ensure that the kettle is not covered during the boil to prevent the volatile containing steam condensing and flowing back into the wort. Commercial kettles often contain a trap in the flue to prevent this from occurring.
Colour and flavour addition
The intense heat generated during boiling promotes various chemical reactions that contribute to the colour and flavour of the beer. Most of these are reactions involving sugars that are caramelised or complex with proteins in Maillard reactions to from dark compounds that influence beer colour. The degree of colour and flavour formation is influenced by the manner in which the heat is applied to the wort. Directly fired kettles can create large amounts of these compounds because of the high temperatures at the point of heat application. This scorching often adds specific character to the beer, but can problem because the scorched wort material may adhere to the base of the kettle and prevent effective heat transfer. For this reason kettles must be cleaned throughly to prevent build up of debris on heating elements.
Monday, January 5, 2009
Clearly Problematic
My personal sulphuric bugbear is the odour and flavour from light struck hops, more commonly known as skunking. It irritates me because it is the most easily preventable of all the invasive sulphur tints that plague beer - just stick the bloody beer in a brown bottle. The chemistry of skunking can be seen below. I love organic chemistry, but it's not for everyone and I am sorry for inflicting it on those who do not share my interest. The photolytic reaction to the left/down is the cause of my woes, the result of which is isopentyl mercaptan - the classic skunky smell. The top reaction running left to right is some clever brewing scientist's bright idea to prevent the breakdown of the hop alpha acid and remove the production of unpleasant sulphur
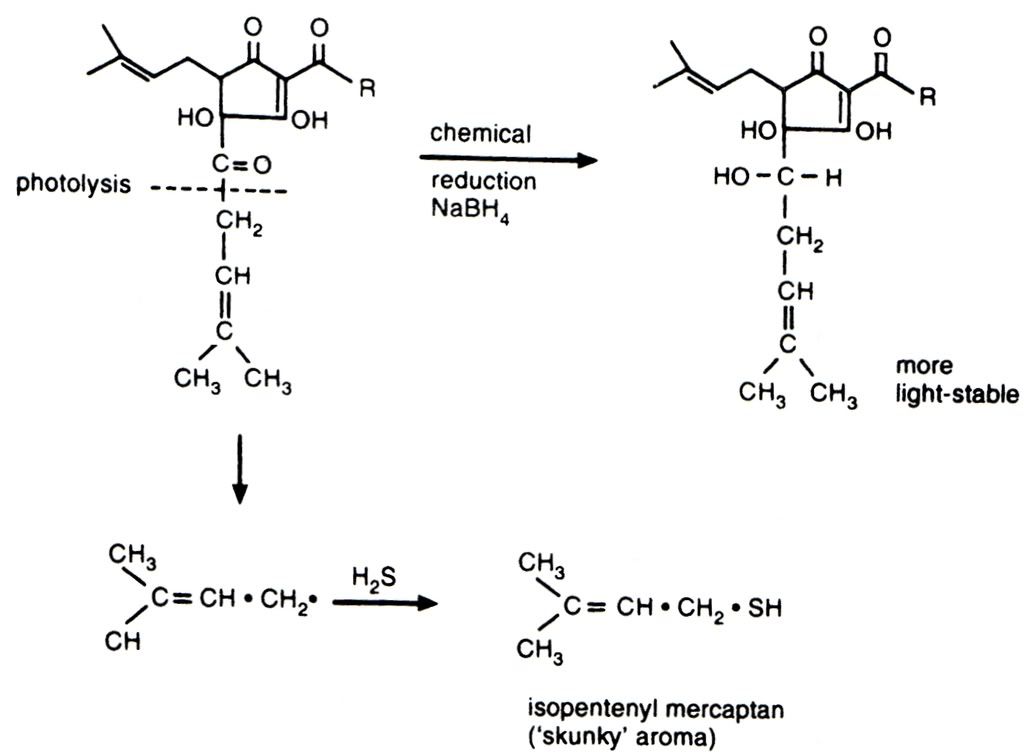 compounds. These products are called reduced alpha acids (reduction is a chemical process involving the donation of electrons to a molecule). These reduced compounds do not suffer from light strike and also have the added bonus of providing more bitterness than an equivalent measure of standard alpha acids. Curiously they also promote far better foam stability, but at the risk of the foam looking rocky and artificial.
compounds. These products are called reduced alpha acids (reduction is a chemical process involving the donation of electrons to a molecule). These reduced compounds do not suffer from light strike and also have the added bonus of providing more bitterness than an equivalent measure of standard alpha acids. Curiously they also promote far better foam stability, but at the risk of the foam looking rocky and artificial.I stopped buying beer in clear bottles quite some time ago because of the unpleasant smell and flavour it suffers from. This is shame because there are quite a number of good beers sold in clear glass bottles. The difference between a beer in its intended state and the sorry condition it reaches us in clear glass has been highlighted to me on a number of occasions. The first was Marston's Old Empire IPA, an unremarkable beer in the bottle that tastes just like every other light struck beer, but a pint of it from cask at th
 e GBBF proved entirely different and not just because of the cask/bottle divide. The most striking difference I have experienced was Bishop's Finger from a bottle that had been kept under wraps away from mischievous photons. It is a true strong malty richly hopped ale in this condition, but tastes like every other ale in a clear bottle when exposed to light. Happily I can say the same for this particular bottle of 1698 because the people at CAMRA must have kept it from the light during processing, and I, hopeful that they had done so, stashed it in the cupboard in the hope that it might be free from light struck off flavours. Thankfully it has not a hint of skunking. Instead it is a wonderful strong ale with full, sticky body and satisfying minerally English hops. I do not know why Shepherd Neame put all their beer in clear bottles. It seems crazy to me, especially with the generous hopping that is typical in most of their ale.
e GBBF proved entirely different and not just because of the cask/bottle divide. The most striking difference I have experienced was Bishop's Finger from a bottle that had been kept under wraps away from mischievous photons. It is a true strong malty richly hopped ale in this condition, but tastes like every other ale in a clear bottle when exposed to light. Happily I can say the same for this particular bottle of 1698 because the people at CAMRA must have kept it from the light during processing, and I, hopeful that they had done so, stashed it in the cupboard in the hope that it might be free from light struck off flavours. Thankfully it has not a hint of skunking. Instead it is a wonderful strong ale with full, sticky body and satisfying minerally English hops. I do not know why Shepherd Neame put all their beer in clear bottles. It seems crazy to me, especially with the generous hopping that is typical in most of their ale.As an aside, in the above photolytic chemical reaction it is the presence of the oxygen atom and double bond that is responsible for light strike. Oxygen is very electronegative which means it really pulls on the electrons in the bond with the lower carbon atom. This weakens the bond and allows the lower fraction of the molecule to be cleaved off by an energetic photon. This same electronegativity is responsible for all life on earth in as much as it is the reason why a small molecule like water is a liquid at ambient temperature. So I'm left with a dilemma; all life on earth or skunk free beer?
Wednesday, November 19, 2008
American? Heaven Forbid
 It is always fun delving into the lucky dip that is the CAMRA Beer Club quarterly delivery. I did well this time with XB Bluebird Bitter from the Coniston Brewing Co. It describes itself as an English pale ale with American aroma hops, but "not too much mind, or we might have an American pale ale on our hands". It need not worry about that, it has a bit to go before things stray into APA territory, but it is a tasty beer with distinct citrus hop notes in the American fashion but strange because it is carbonated like an English ale and the combination is a little unusual. The colour is very appealing, and a triumph of bottle conditioning in that it is very easy to pour without agitating the yeast sediment and has perfect condition.
It is always fun delving into the lucky dip that is the CAMRA Beer Club quarterly delivery. I did well this time with XB Bluebird Bitter from the Coniston Brewing Co. It describes itself as an English pale ale with American aroma hops, but "not too much mind, or we might have an American pale ale on our hands". It need not worry about that, it has a bit to go before things stray into APA territory, but it is a tasty beer with distinct citrus hop notes in the American fashion but strange because it is carbonated like an English ale and the combination is a little unusual. The colour is very appealing, and a triumph of bottle conditioning in that it is very easy to pour without agitating the yeast sediment and has perfect condition.Bottle conditioning is a tricky thing to do well. Brewers are given a number of options in the bottling of live beer and it's hard to know which is best. One question the brewer must address is how the yeast is to be provided with enough extract to condition the beer. One option is to halt the fermentation by cooling the wort before the yeast have used all the sugars. The beer can then be put into cask and bottles where the yeast continue to ferment the wort when things warm up and provide a degree of carbonation in the product. That's very bloody hard to judge, I imagine. Another option is to let the beer ferment out and then add some priming sugar to the bottling tank. This appeals to me as a home brewer because it is exactly what we do and works very effectively, but the volumes involved at the commercial scale might be impractical. Also, there are specific bacteria that just love priming sugar and ruin beer. Another appealing option is to add a measure of freshly fermenting beer to the bottling tank. Termed 'krausening' this has the double benefit of supplying extract for conditioning and also an infusion of fresh yeast that will carbonate the beer in peak condition, but again could prove difficult because the primary fermentation of one batch must be carried out with the bottling of another batch in mind.
Yeast counts are also a problem because too much yeast in the bottle will either give a sludge of dead yeast in the bottom of the bottle or a yeast layer that lifts too easily and fogs up the pint. Some brewers opt to reduce the yeast content to half a million cells per millilitre by cooling the beer in the fermenter thereby encouraging most of the yeast to drop out of the beer. Others roughly filter the beer to remove all the yeast, but leave the tastier components, and then re-introduce a specific amount of yeast to take care of things in the bottle. The yeast count in the bottle must be sufficient to allow conditioning of the beer within a few weeks and also enough to produce a thin uniform film over the bottom of the bottle preventing slippage of the sediment, providing ease of pour.
There is no doubt that it is easier, if a little more expensive, to run your beer through a fine filter to stabilise it and not worry about all these complex considerations, but I am glad that a great many brewers in Britain have persevered with this tricky business to provide us with live, flavourful beers.
Wednesday, November 12, 2008
Black Lightning & the DMS Issue
 This quarter's CAMRA Beer Club belatedly arrived yesterday, just as I was starting to get a little concerned about it. A previous delivery had gone missing in the post so I get a little nervous when it's overdue. This month's motley crew of real ale look quite promising. Among them is Entire Stout by the Hopback Brewery of Wiltshire. So effective is the marketing of their golden ales it is very strange indeed to see a black beer coming from one of their bottles. I have enjoyed Summer Lightning many times in the past both bottled in Ireland and on cask in England and each time thought it a wonderful beer. I was surprised when Tim said he noted a distinct DMS note to it, and swiftly commented that DMS in unlikely in a beer brewed with pale malts. I had to eat my words shortly afterwards when I tried a bottle out of curiosity and was met with a strong vegetal note, typical of high DMS levels.
This quarter's CAMRA Beer Club belatedly arrived yesterday, just as I was starting to get a little concerned about it. A previous delivery had gone missing in the post so I get a little nervous when it's overdue. This month's motley crew of real ale look quite promising. Among them is Entire Stout by the Hopback Brewery of Wiltshire. So effective is the marketing of their golden ales it is very strange indeed to see a black beer coming from one of their bottles. I have enjoyed Summer Lightning many times in the past both bottled in Ireland and on cask in England and each time thought it a wonderful beer. I was surprised when Tim said he noted a distinct DMS note to it, and swiftly commented that DMS in unlikely in a beer brewed with pale malts. I had to eat my words shortly afterwards when I tried a bottle out of curiosity and was met with a strong vegetal note, typical of high DMS levels.I might take a minute to jot down a few notes on dimethyl sulphide (DMS) to clarify why it is unusual to note it in some beer rather than others. It is a sulphur compound as the name suggests, and like mo
 st sulphur compounds is quite unpleasant smelling. It is a common feature of some lagers because lager malt contains large amounts of the precursor to DMS, S-methyl methionine (SMM), but the levels are low enough to add a distinct character to the lager which is often desirable. SMM is broken down into DMS by heat which in brewing occurs during the boil and during kilning in the production of malt. Thankfully DMS is quite volatile so it is driven off to the atmosphere during these processes. Pale ale malt is kilned at a high temperature compared to lager malt and as a result most of the DMS is driven off leaving very little in the finished malt. This explains my surprise that a golden ale brewed presumably with pale ale malt would have a stro
st sulphur compounds is quite unpleasant smelling. It is a common feature of some lagers because lager malt contains large amounts of the precursor to DMS, S-methyl methionine (SMM), but the levels are low enough to add a distinct character to the lager which is often desirable. SMM is broken down into DMS by heat which in brewing occurs during the boil and during kilning in the production of malt. Thankfully DMS is quite volatile so it is driven off to the atmosphere during these processes. Pale ale malt is kilned at a high temperature compared to lager malt and as a result most of the DMS is driven off leaving very little in the finished malt. This explains my surprise that a golden ale brewed presumably with pale ale malt would have a stro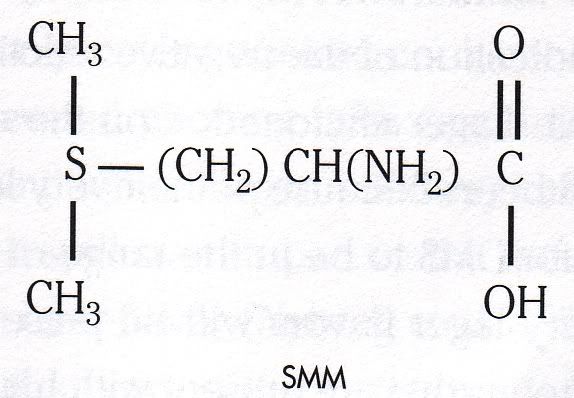 ng DMS element to its character. The smell of DMS appears to vary with its concentration. Bamforth describes it as 'cat urine' which I can agree with because this is the distinct smell I get from Heineken, particularly in bottles. This might sound like I'm slagging Heineken off, but in fact a high DMS aspect in a lager suggests that it is made with a great deal of malt, rather than cheaper adjuncts that might thin out the body. The other smell commonly used is cooked corn, which I must confess I have never experienced. Another common smell is a rotting vegetable like odour, clearly outlined to me during a brewing course I undertook during which a concentrated DMS solution was passed around for us to smell. It had the unmistakable odour of cabbage left to rot in the drain of a sink after the washing up is done.
ng DMS element to its character. The smell of DMS appears to vary with its concentration. Bamforth describes it as 'cat urine' which I can agree with because this is the distinct smell I get from Heineken, particularly in bottles. This might sound like I'm slagging Heineken off, but in fact a high DMS aspect in a lager suggests that it is made with a great deal of malt, rather than cheaper adjuncts that might thin out the body. The other smell commonly used is cooked corn, which I must confess I have never experienced. Another common smell is a rotting vegetable like odour, clearly outlined to me during a brewing course I undertook during which a concentrated DMS solution was passed around for us to smell. It had the unmistakable odour of cabbage left to rot in the drain of a sink after the washing up is done.Getting back to the beer in hand, it's a nicely bitter stout with some chocolate in there somewhere, which really comes through during the swig when your nose is buried in the glass. The roasted barely is clearly evident to me at the moment because of late I have been drinking my own dark beer which is without any roasted barley at all. The bottle states it is suitable for vegans because it is not fined and has no residue of marine swim bladders in the form of isling glass. It seems common enough practice for brewers not to fine or filter their stouts, the Porterhouse don't bother either because stouts will hide any haze issues. All the better for us because nothing is stripped from the beer. It might not be so good for a work colleague of mine with a yeast sensitivity who asked me a few years ago if there was any yeast in commonly available draught beers. In my then ignorance, I told her no, it is all filtered, except for wheat beer, so she'd be safe enough. Let's hope she doesn't get a hankering for decent stout anytime soon...
Sunday, November 2, 2008
Water water everywhere
Every brewer knows that the quality of the water used in the production of beer is of paramount importance. Water can provide direct flavour effects and also indirect effects upon mash, wort boiling and fermentation. The water we use for brewing is a mix of various inorganic ions which stem from the environment the water passed through during its journey to your tap or well. The concentrations of these ions vary depending upon geographical location and there can be no doubt that some sources of water are better for brewing than others, but as brewers we have a degree of influence over the make up of the water that enters the mash tun.
The common ions in water and their direct influence on flavour are:
Sodium: contributes a salty taste at a concentration of 150 to 200 mg/l and may be harsh at levels greater than 250 mg/l. At lower levels (up to 100 mg/l) sodium ions can produce a sweetening effect in conjunction with chloride ions.
Potassium: can be salty at concentrations greater than 500 mg/l. Beer is high in potassium which is extracted from malt but it is essentially flavour neutral at these levels. Potassium chloride can be considered as a source of chloride instead of sodium chloride if sodium levels are too high.
Magnesium: can contribute a bitter and sour flavour if above 70 mg/l, though this effect is dependant upon a balance with calcium ions.
Calcium: flavour neutral except for its effects on Mg influence
Iron: gives metallic and astringent flavours at levels as low as 0.5 mg/l and even lower in lighter beers.
Chloride: gives fullness and sweetness with optimal effects between 200 to 400 mg/l
Sulphate: imparts dryness and astringency and increase bitterness. Optimal levels are found from 200 to 400 mg/l
Hydrogen: The effect of hydrogen ions is felt through influence on beer pH. At pH values below 4.0 beer tastes more sharp and acidic and perceived bitterness is increased. Values below this cause increased metallic after taste. Above pH 4.0 effects on mouth coating occur resulting in greater biscuit and toasted flavours noted. Above pH 4.4 mouth coating increases with soapy and caustic characters develop.
A number of brewing texts refer to the importance of chloride/sulphate balance because of the antagonistic effects of these two ions. Studies have shown a shift from 1:1 to 2:1 chloride:sulphate increased the perceived sweetness while a shift in the ratio towards sulphate increased perceived bitterness and astringency. Additions of salts such as calcium chloride and calcium sulphate (gypsum) provide brewers with a means of adjusting this balance thereby adjusting the flavour to suit the style of beer brewed. The indirect effects of ions in water are probably of more significance to the quality and flavour of beer than the direct effects they contribute. These indirect effects are manifested through the interaction of ions with malt constituents and wort components. The main direct effects can be divided into:
Yeast requirements: Fermentation is an immensely complicated combination of enzyme reactions that ultimately result in the production of ethanol, carbon dioxide and small quantities of flavour compounds from the anaerobic metabolism of maltose and other malt constituents. In order for these reactions to go ahead smoothly without the production of undesirable flavour compounds yeast must have all the nutrients required to maintain these essential metabolic pathways. Water provides some these essential ions while others are derived from the grist.
Effects on malt enzymes: Suitable water provides a good environment for the action of malt enzymes during mashing which ensures full extraction of fermentables from the grist. The most significant contribution from water is calcium ions which stimulate and protect malt amylases, in particular protecting them from heat inactivation.
Effects on colloidal stability: The main contribution water makes to colloidal stability is through the addition and action of calcium. Calcium levels of at least 50 mg/l are required for good yeast flocculation while at least 100 mg/l are required for good break formation. During break formation calcium forms complexes with proteins, polyphenols and hop constituents aiding their removal from the wort. This greatly helps with wort clarification and can reduce haze potential in the beer. A further action of calcium involves removal of oxalate in the form of calcium oxalate. Oxalate stems from the malt and too much in the finished beer causes gushing upon opening.
Them beers them beers need calcium
Something worth bearing in mind with respect to calcium concentration is the different stages of the brew that various amounts are required. If you have assessed that a certain addition of calcium sulphate will bring your calcium levels up to the level required to mash effectively, the question must be asked how much of the calcium is left behind in the spent grains, and do you have sufficient calcium left for both effective boiling and good yeast flocculation. It is suggested that calcium levels are depleted by 50 to 60% due to losses in the spent grain. Therefore sufficient calcium should be added to maintain calcium levels throughout the rest of the brewing steps.
Tuesday, July 15, 2008
I'm Afraid It's Terminal
 My most recent attempt at propagating a yeast starter has proved disastrous. I had hoped to produce a large cell mass and use my nerdy beer equipment to do a cell count and pitch a known number of cells per mililitre of wort, but this plan came to an abrupt end when I removed the foil from the flask to take a sample for counting and was met with the unmistakable smell of contamination. In case you don't know, this smell is identical to the phenolic fluid that the local council use to wash out lanes and such that have been used as a communal urinal by non community minded individuals. If you have been spared the unpleasant experience of passing a lane that has been washed out thusly, the classic association with this smell is hospitals in the old days when everything was scrubbed with phenol and super bugs gave them a wide birth.
My most recent attempt at propagating a yeast starter has proved disastrous. I had hoped to produce a large cell mass and use my nerdy beer equipment to do a cell count and pitch a known number of cells per mililitre of wort, but this plan came to an abrupt end when I removed the foil from the flask to take a sample for counting and was met with the unmistakable smell of contamination. In case you don't know, this smell is identical to the phenolic fluid that the local council use to wash out lanes and such that have been used as a communal urinal by non community minded individuals. If you have been spared the unpleasant experience of passing a lane that has been washed out thusly, the classic association with this smell is hospitals in the old days when everything was scrubbed with phenol and super bugs gave them a wide birth.I was miffed with this turn of events because I have never suffered a spoiled yeast starter before, but know exactly where the contamination crept in. My usual method of aeration is to vigorously sha
 ke the flask of wort in much the same way I strenuously shake the 25 litre carboy of wort before fermentation kicks off. This time, however, I took the advice of an American website which suggested shaking smaller volumes in a separate vessel and pouring into the primary flask. I was keen to get as much oxygen into the wort as possible, but was all too aware that this method required the wort contacting far more surfaces and atmosphere than I like. I'll be sticking to the tried and tested next time.
ke the flask of wort in much the same way I strenuously shake the 25 litre carboy of wort before fermentation kicks off. This time, however, I took the advice of an American website which suggested shaking smaller volumes in a separate vessel and pouring into the primary flask. I was keen to get as much oxygen into the wort as possible, but was all too aware that this method required the wort contacting far more surfaces and atmosphere than I like. I'll be sticking to the tried and tested next time.The pictures with this post are microscope shots of the contaminated starter. I must admit to being a little confused by it all because my experience with yeast morphology is limited. What we are looking at is either the smaller desired yeast strain along with a typically larger wild yeast strain that caused the nasty smell, or we are looking at the larger desired yeast strain accompanied with smaller bacterial cells. I'm not certain, but there's definitely something in there that shouldn't be.
Monday, July 14, 2008
Under Presssure
 I love beer served from my pressure barrel. It even beats cask dispensed to my mind, though I suppose they are one in the same in many respects; both are naturally carbonated and served at around 12 degrees centigrade. The manner in which the beer is discharged from the vessel is different though, and perhaps this makes all the difference. When I fill the barrel I prime it to around 3 or 4 parts CO2, a shocking degree of carbonation, just the thought of which makes my trigeminal nerve ache and no doubt dedicated real ale drinkers cry into their pints. However, this vast amount of CO2 merely propels the beer from the barrel and provides a degree of turbulence from the tap which brings a satisfying head to the beer. After dispense I'd wager the beer contains little more than 1 to 1.5 parts CO2, and feels perfect on the tongue.
I love beer served from my pressure barrel. It even beats cask dispensed to my mind, though I suppose they are one in the same in many respects; both are naturally carbonated and served at around 12 degrees centigrade. The manner in which the beer is discharged from the vessel is different though, and perhaps this makes all the difference. When I fill the barrel I prime it to around 3 or 4 parts CO2, a shocking degree of carbonation, just the thought of which makes my trigeminal nerve ache and no doubt dedicated real ale drinkers cry into their pints. However, this vast amount of CO2 merely propels the beer from the barrel and provides a degree of turbulence from the tap which brings a satisfying head to the beer. After dispense I'd wager the beer contains little more than 1 to 1.5 parts CO2, and feels perfect on the tongue.Over carbonation is disasterous in a beer that doesn't warrant it. When it's a part of the experience, such as a tangy refreshing wheat beer I enjoy it greatly, but fizzy ale is just terrible. I find the carbonic flavour overpowers just about everything and the sting on the tongue is not welcome at all. The problem is, I find this happens quite often with many commercial ales, but if so many breweries think this is a suitable way to serve ale, perhaps it is the accepted norm and I am merely being overly sensitive, much like my dislike of beer sold in clear glass bottles ( a whole other post, that one...). So many brewers continue to do both with seemingly little affect on their popularity that the vast majority of ale drinkers must be happy with it.
Thursday, April 24, 2008
Stir it up
 As I mentioned in my last post I used a liquid yeast to ferment my latest brew. I had stayed away from liquid yeast for the last while because I didn't think I was getting the best from them. Coupled with this was the extra expense involved because liquid yeast strains are up to four times the cost of dried yeast sachets so I wanted to make the most of the investment. To this end I invested in some equipment which should help in the propagation of yeast from starter packs. Working in a lab brings to my attention all manner of equipment that is of great use for home brewing but might seem a little excessive to the home brewer who is not familiar with them. The simplest of this equipment is a selection of flasks that are well suited to yeast propagation, which when coupled with a magnetic stirring plate are a very effective way of persuading yeast to grow.
As I mentioned in my last post I used a liquid yeast to ferment my latest brew. I had stayed away from liquid yeast for the last while because I didn't think I was getting the best from them. Coupled with this was the extra expense involved because liquid yeast strains are up to four times the cost of dried yeast sachets so I wanted to make the most of the investment. To this end I invested in some equipment which should help in the propagation of yeast from starter packs. Working in a lab brings to my attention all manner of equipment that is of great use for home brewing but might seem a little excessive to the home brewer who is not familiar with them. The simplest of this equipment is a selection of flasks that are well suited to yeast propagation, which when coupled with a magnetic stirring plate are a very effective way of persuading yeast to grow.To see why these pieces of equipment are useful in propagating yeast we must venture into a little yeast biochemistry and metabolism for an explanation. Yeast are wonderful survival machines with the ability to survive in both aerobic and anaerobic environments. In an anaerobic environment such as brewers wort yeast use the sugars to produce energy with ethanol produced as a glorious waste product. If however yeast find themselves in an aerobic environment they respire much like us producing carbon dioxide and water. With this is mind we can see that it is very important that the environment that yeast finds itself in during beer fermentation must be anaerobic otherwise we would have the horrifying situation of no ethanol production, vast amounts of yeast cells and a strange liquid that couldn't really be described as beer. Thankfully, there is no way that brewers can accidentally aerate to such as extent that ethanol production is completely inhibited. However at the beginning of fermentation a certain degree of oxygenation is essential to set the yeast up for the work they have ahead. Before pitching, yeast is usually stored for a period during which it has to rely upon its own reserves to survive. These reserves are rapidly depleted during storage and yeast is in no position to effectively ferment beer when it is pitched. A ready supply of oxygen at the beginning of fermentation is necessary for the yeast to replenish itself and and produce sterols to make the cell wall permeable to the wort constituents. Also during this period there is an increase in the number of cells, as cell proliferation occurs at the expense of ethanol production in the aerobic environment. It is after this stage that things kick off and a vigorous fermentation ensues with lots of gas production accompanied by copious foam and ethanol, of course.
make the cell wall permeable to the wort constituents. Also during this period there is an increase in the number of cells, as cell proliferation occurs at the expense of ethanol production in the aerobic environment. It is after this stage that things kick off and a vigorous fermentation ensues with lots of gas production accompanied by copious foam and ethanol, of course.
With this little bit of information in mind the objective of yeast propagation can be laid out. Liquid yeast for home brewing is in a depleted state, ready and waiting for a chance to grow. The number of cells in the average yeast pouch is insufficient to pitch, so a propagation step is needed. This step is essentially the same as the first aerobic stage of fermentation and this is where my equipment comes into use. Sterile aerated wort is placed in a flask and the depleted yeast pouch is added to the wort where it is continually mixed on the magnetic stirrer. The aim of this exercise is to produce as many yeast cells as possible to meet the pitching rate required for the gravity of the beer, which is easily done in an aerobic environment where the yeast will proliferate rather than produce ethanol. The mixing plate keeps the cells in contact with the wort ensuring maximum use of the sugar available, and also mixes the oxygen making it available to the yeast. Studies suggest that greater cell counts can be achieved with this method and it is also a hell of a lot handier than having to shake the flask every time you walk by it.
Generally a much faster start to fermentations can be had this way because the yeast is ready to go when pitched which has many advantages in the home brew environment, the main one of which is not having a big bucket of nutritious sweet wort laying about the place just screaming out for some opportunistic bug to come along and ruin. Once the fermentation kicks off the pH starts to drop rapidly creating an acidic environment that suits only the hardiest of bugs (and they sadly are quite common) and the increase production of ethanol contributes further to the inhospitable environment.I'll leave it at that because the other great aspect to using liquid yeast is the complexity they bring to beers, which is often greater than that of dried strains. But this is a touchy subject with home brewers because some believe that dried strains are just as good. I don't agree, and that debate is worthy of its own post at a later time.
Monday, March 31, 2008
Distillers Branching Out
 I spotted this beer a number of weeks ago in the off license beside where I work, but passed up the opportunity to purchase it on a number of occasions mainly I suppose because of its expense and hefty ABV. I just wasn't in the mood for a heavy beer, and besides this establishment was knocking out the mighty tasty Brooklyn Lager a two quid a pop, so I loaded up on that because it is one of my favourite session lagers.
I spotted this beer a number of weeks ago in the off license beside where I work, but passed up the opportunity to purchase it on a number of occasions mainly I suppose because of its expense and hefty ABV. I just wasn't in the mood for a heavy beer, and besides this establishment was knocking out the mighty tasty Brooklyn Lager a two quid a pop, so I loaded up on that because it is one of my favourite session lagers.It is grandly dubbed 'Tullibardine 1488, Majestic Whisky Ale', and is brewed in the Tullibardine distillery, which is interesting to me because I am not aware of any other distilleries that have branched out into brewing beer. Sure, there are any number of breweries that have pilfered whisky and bourbon barrels from distilleries and stuffed their beer in them for a few months, but it's unusual for a distiller to bother himself with brewing beer. In some respects it's not that big a step, after all, the distiller has all the malt he needs, understands how to conduct a mash and handles yeast with skill. All he need do is get a kettle to boil in, purchase some hops and grab a brewing strain of yeast that won't over attenuate the wort (or wash, as distillers call it). I imagine there are any number of vessels lying about the place suitable for fermenting in, and of course he has a ready supply of fragrant whisky barrels.
Having studied a bit about the chemistry of whisky maturation I can see what these guys are driving at. Whisky casks must be made of oak, and usually stem from either Spain where they matured sherry in a previous life, or North America where they did their bit for Jack Daniels or Jim Beam before being bought up by Scottish distillers to mature their wonderful single malts. This goes a long way to explaining why typical North American bourbon is as rough as old boots compared to Scotch. While bourbon is not made from high quality malted barley - and this certainly affects the flavour, the raw woody aromas stem from the first time use of the charred oak barrel. The spirit is put in the barrel for a few years, and the ethanol extracts all manner of interesting compounds like tannins and lactones from the charred wood. Only once this process has occurred is the barrel suitable for the long maturation of Scotch whisky. Many of the rough elements are extracted by the bourbon leaving a more subtle and smoother flavour in the Scottish product. For some of the very flavourful and heavy single malt whiskies old sherry casks are used which are impregnated with potent flavour compounds derived from sherry maturation. During whisky maturation the spirit is often a 60% ABV and readily extracts compounds from the wood, so perhaps the hefty 7% ABV of this beer is intended as a means of getting a bit more character from the beleaguered oak barrels.
The beer itself is the colour of a rich single malt whisky, but the whisky character is not that pronounced; wine seems to be the dominant flavour suggesting that old sherry casks were used to mature the whisky that occupied the casks before the beer. The alcohol content is evident, with a satisfying warmth all the way down the throat. It perhaps resembles whisky more in this respect than any reference to flavour. There is little in the way of hop bitterness, and just enough malt to hold it all together. Interestingly, there is also a distinct lactic tang which likely stems from micro fauna in the cask, but how well bacteria can live in a cask filled with whisky is something I am unsure off. It is a light bodied beer for its alcohol content, and initially feels a little like a Belgian triple, but the over all impression is tart and refreshing, which is surprising for such a strong beer.
Sunday, March 30, 2008
Go forth and Flocculate
I read up on flocculation recently in order to write an assignment for my brewing course and learned a number of interesting things in the process. I knew little of the mechanism of yeast flocculation, and was interested to learn that it appears to one of those things that is of little use to the yeast themselves, but crucial to the production of the beer we all love so much. First of all, flocculation was mistakenly attributed to individual yeast cells merely falling from suspension under the action of gravity and collecting in a smelly mass at the bottom of the fermenter. Studies have shown that flocculation actually involves complex interaction between groups of cells that clump together at very specific times in the fermentation cycle. Usually this is during the stationary phase of yeast growth when they have carried out the sterling work of transforming the sugars into ethanol.
In order to understand why the yeast decide to flocculate during this phase of fermentation we must turn to the yeast cell wall and look at the manner in which the cells stick together. The current thinking in yeast studies refers to the 'lectin theory of flocculation'. Lectins are long chain like molecules that stick out from the cell and bind to receptors on neighbouring cells. The receptors are also binding points for sugars, which wort is awash with. This is why most yeast strains will not flocculate before all the sugars are gone; the receptors are tied up with sugars and the long lectin molecules cannot gain access and hook up with the neighbouring cell. From a practical point of view this might explain why that beer you want for the stag night next weekend is refusing to clear; there is just too much priming sugar floating around and the yeast hasn't managed to get through it all yet.
Further practical issues with flocculation involve either premature flocculation, or a stubborn refusal to flocculate at all. Flocculation too early in the fermentation process results in under attenuation of the beer, with resultant alteration to expected character through excess residual extract and drop in ABV. Refusal to flocculate causes over attenuation, and a hazy beer if you're a home brewer, or a massive load on the filtration system if you are a commercial brewer.
There are a number of ways to reduce possible problems with flocculation, the most obvious of which is to avoid those strains of yeast that are known to hang about longer in the beer than is perhaps useful, or drop like a stone at the first opportunity. Certain beer styles require very specific flocculation characteristics to achieve distinctive flavour and body. The strain used by Fuller's for their ESB comes to mind because it flocculates very readily, and this gives the familiar full body caused by high residual extract.
A further way to maintain expected flocculation patterns is to ensure there is sufficient calcium present in the wort. Calcium is absolutely critical for all flocculation as well as mashing and break production, so add it to the brewing liquor if it's in short supply.